Recent Trend in Chemistry
Vitamin C-induced CO2 capture enables high-rate ethylene production in CO2 electroreduction
2 Jan 2024 Nature Communications (Open access online Journal) 15 Edition article number 192 has
published this, it is the work of Jongyoun Kim, Taemin Lee, Minkyoung , Jungsu Eo and others.
As the requirement of energy and continuous fuel supply is concern, we are still struggling, this article shows the way how to prepare carbon-based fuels with the help of Vitamin C, Cu nano wire, CO2 and electric field.
The electrochemical CO2 reduction reaction (CO2RR) to form value-added fuels and feedstocks is a promising route to achieve carbon neutrality and long-term energy storage. The development of CO2RR electrocatalysts has led to advances in selectivity for multicarbon (C2+) chemicals such as ethylene (C2H4) and ethanol (C2H5OH) with high energy density and a high market price.
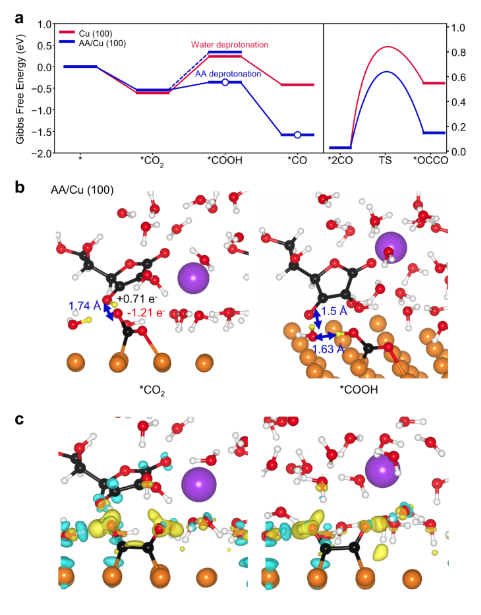
Here, we report molecularly enhanced CO2-to-*CO conversion and *CO dimerization for high-rate C2H4 production using ascorbic acid (AA). AA, also known as vitamin C, has been widely used as a reducing agent and antioxidant in nanomaterial synthesis and biochemical purposes. When we store fruits to preserve AA, maintaining a CO2-deficient environment is essential because AA can react with CO2 and be oxidized to dehydroascorbic acid (DHA) with proton and electron donation. Furthermore, AA has been utilized for CO2 capture in homogeneous catalysis approache. Inspired by this AA/DHA redox principle and CO2 capture property, we exploited AA as a promoter to capture CO2 near Cu, increase the *CO coverage and ensuing *CO dimerization on the surface of heterogeneous Cu catalysts.
To employ AA in heterogeneous catalysis with aqueous electrolytes, we pursued a strategy to immobilize water-soluble AA on electrocatalysts and achieve redox reversibility. We designed AA-augmented Cu nanowires (CuNWs) by applying graphene quantum dots (GQDs), which contain −OH and −COOH groups, as a mediator to anchor AA on the Cu surface with an ionomer. This nanoconfined AA on CuNW enhanced the CO2-to-*CO conversion during the CO2RR and resulted in high C2H4 productivity of heterogeneous Cu electrocatalysts. Unlike pristine CuNW (p-CuNW), which mainly produced C2H4 at low potential, CuNW with AA nanoconfined by GQDs (cAA-CuNW) boosted CO production over a similar potential range. As the potential increased for the high-current-density CO2RR, enriched CO formation in cAA-CuNW was dramatically converted to C2H4, while the main electrolysis product of p-CuNW was hydrogen (H2) because of limited CO2 mass transport. We found that this enables efficient CO2RR even in low CO2 concentrations, which can be extended to the CO2RR of flue gas. In situ Raman spectroscopy and operando X-ray absorption spectroscopy (XAS) studies enabled us to verify the effect of nanoconfined AA for inducing a high degree of *CO coverage and binding control between atop-bound CO (COatop) and bridge-bound CO (CObridge) on the reconstructed CuNW during the CO2RR. Grand canonical density functional theory (GC-DFT) revealed that the redox of AA/DHA enabled efficient electron/proton transfer to CO2 and multiple hydrogen bonding sites of AA, thereby improving CO2-to-*CO conversion and *CO dimerization on Cu.
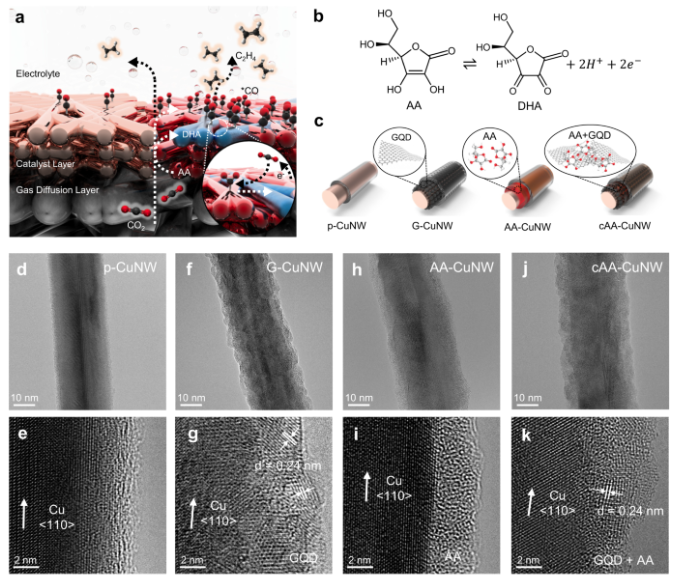
a Schematic of enhanced CO2-to-*CO conversion and *CO dimerization in cAA-CuNW for high-rate C2H4 production. b Redox of AA and DHA for CO2 capture. c Schematic illustration of surface modification of CuNWs with GQD, AA, and nanoconfined AA on GQDs. An ionomer is coated on the outer surface of CuNWs during the fabrication of the GDE. TEM (top) and HR-TEM (bottom) images of (d, e) p-CuNW, (f, g) G-CuNW, (h, i) AA-CuNW, and (j, k) cAA-CuNW.